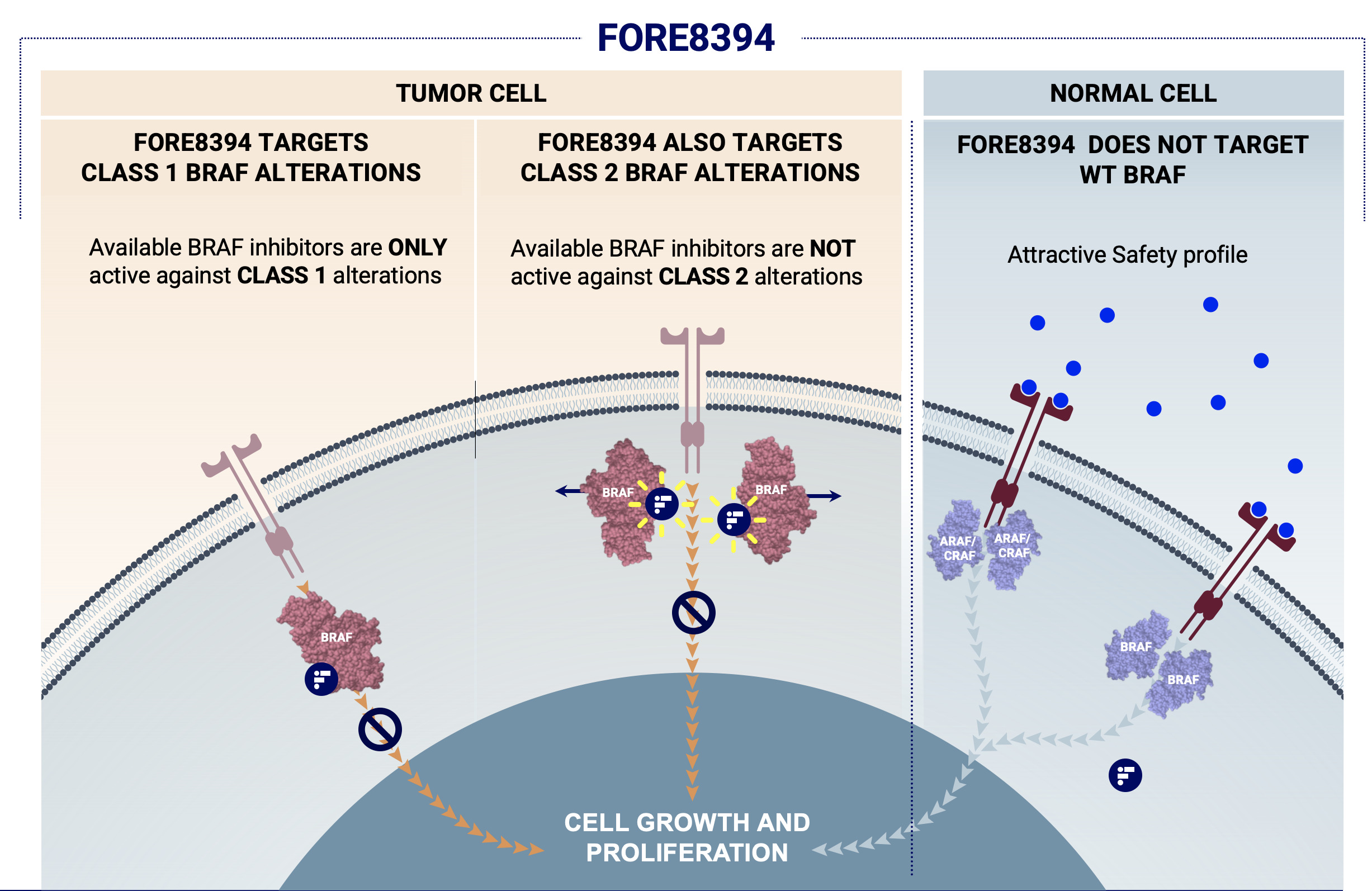
Plixorafenib was designed to target a wide range of BRAF mutations while sparing wild type forms of RAF. Preclinical studies and clinical trials have shown that its unique mechanism of action effectively inhibits not only the constitutively active BRAFV600 monomers targeted by first-generation RAF inhibitors, but also disrupts constitutively active dimeric BRAF class II mutants, fusions, splice variants and others.
Plixorafenib Program

About the program
Plixorafenib (formerly FORE8394, PLX-8394) is an investigational, novel, small-molecule, next-generation, orally available selective inhibitor of mutated BRAF. Its inhibition of ERK signaling makes it a strong candidate for the treatment of tumors driven by class I or II BRAF mutations and fusions.
The U.S. Food and Drug Administration (FDA) granted Orphan Drug designation for plixorafenib for the Treatment of Primary Brain and CNS Malignancies and Fast Track designation for the treatment of cancers harboring BRAF Class 1 (V600) and Class 2 (including fusions) alterations who have exhausted prior therapies.
Beyond BRAFV600 mutations
Paradox-breaker
Unlike first-generation RAF inhibitors, plixorafenib does not induce paradoxical activation of the RAF/MEK/ERK pathway. As a “paradox breaker,” plixorafenib could therefore treat acquired resistance to current RAF inhibitors, and, more generally, yield improved safety and more durable efficacy than first-generation RAF inhibitors.
BRAF-specific dimer-breaker
As a BRAF-specific dimer breaker, plixorafenib selectively inhibits ERK signaling in tumors driven by dimeric BRAF mutants, including BRAF fusions and splice variants, as well as BRAFV600 monomers, but spares RAF function in normal cells in which CRAF homodimers can drive signaling.
Information about our ongoing clinical trial
Fore is running a clinical trial open for patients with advanced cancer with BRAF alterations, including both patients with CNS and solid tumors NCT05503797.