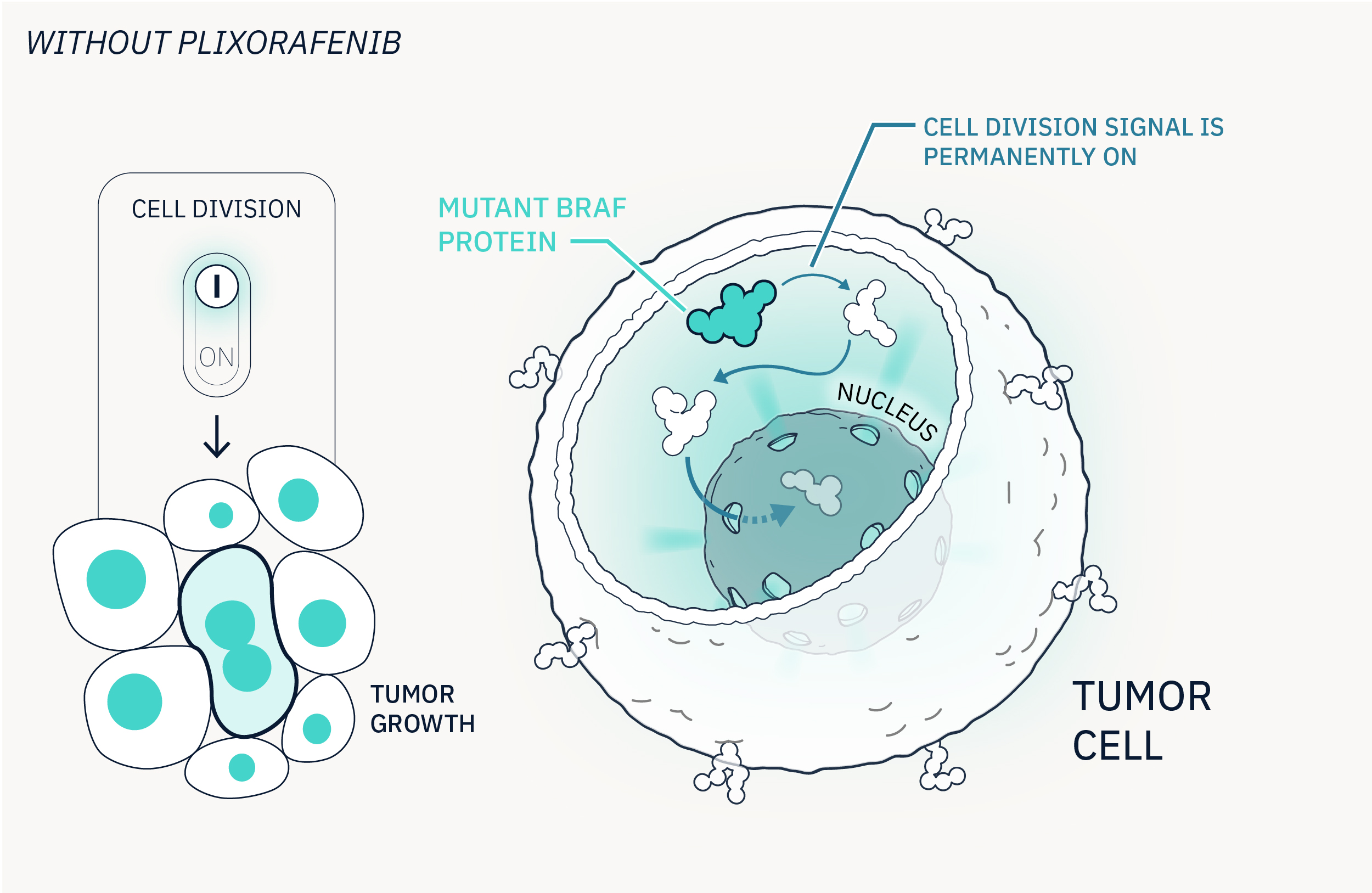
Plixorafenib targets cancer cells with BRAF mutations
Plixorafenib is a small molecule that targets cancer cells with a mutated BRAF gene. Unlike conventional chemotherapy, this targeted therapy spares healthy cells, as their BRAF gene is not mutated.