We aim to provide rapid and high quality access to the right medicines for the right patients with the hardest-to-treat cancers.

KOL Roundtable Discussion on Plixorafenib
Wednesday, October 15, 2025
12:00 pm ET

We are creating a pipeline of precision oncology treatments aimed at patients with unaddressed mutations across well-established oncology targets.
Plixorafenib Program
Our lead program focuses on plixorafenib, an investigational, next-generation BRAF inhibitor designed to inhibit a wide range of BRAF gene alterations in cancer cells beyond those targeted by earlier-generation BRAF inhibitors.

We harness the full power of innovation, within and outside our walls, to deliver high-impact therapies to patients with unaddressed tumor mutations with speed and quality
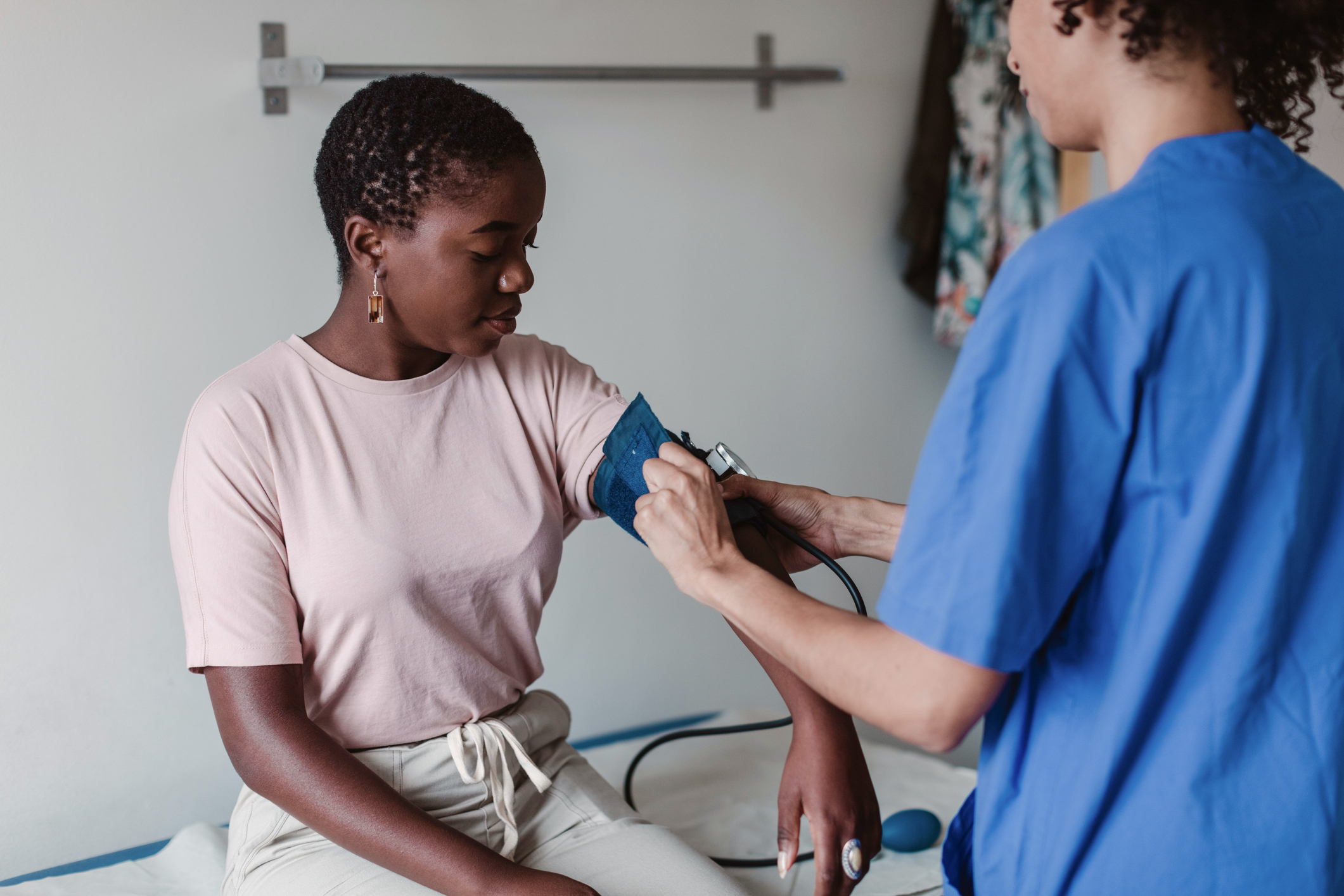
Following strong preclinical and early clinical results, we’re currently running a phase 2 clinical trial
Following strong preclinical and early clinical results, an active phase 2 study is currently underway to assess the efficacy and safety of our lead candidate plixorafenib for previously treated patients with advanced, unresectable solid tumors harboring BRAF alterations.
Plixorafenib is an Investigational Drug and has not been approved by the U.S. Food and Drug Administration or other regulatory authorities.

Exceptional research depends on exceptional people
Fore Biotherapeutics is a virtual Biotech company with expertise and talent across the USA
Latest News









Join a growing leader in the biotech industry.
Partnerships
We put strategic partnerships at the heart of our approach. We focus on in-licensing clinic-ready compounds and can accommodate a wide range of partnering structures.
Investors
Fore is backed by top investors with deep industry knowledge and a successful history of forming some of the world’s leading oncology companies.